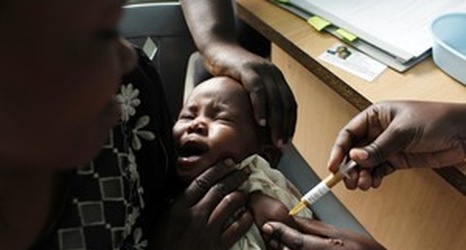
LONDON (AP) — The European Medicines Agency has recommended approving what would be the world’s first licensed malaria vaccine, even though it’s only about 30 percent effective and its protection fades over time.
In a statement Friday, the agency endorsed the vaccine’s use outside Europe, a regulatory process that helps speed new medicines to the market.
The recommendation to license the vaccine, known as Mosquirix and made by GlaxoSmithKline, must still be approved by the European Commission.
The World Health Organization will next consider the evidence and recommend how the shot be used. It’s unlikely donors would pay for the vaccine without WHO’s guidance, especially since it only protects about one-third of the children vaccinated.